Gemtuzumab ozogamicin (GO) is a CD33-directed antibody drug conjugate (ADC) that is re-emerging as an approved therapeutic option in acute myeloid leukemia (AML). Initial in vitro and in vivo data suggested enhanced response to GO in cells with high expression of CD33. However, due to lack of a biologic threshold for CD33 expression, phase III clinical trials treated all patients with GO regardless of expression of CD33. Early data from MRC, COG and ALFA trials demonstrated modest efficacy of GO, with some studies suggesting response in only subsets of patients, including those with distinct cytogenetic and molecular features. Splicing of CD33 transcript is in part regulated by a single nucleotide polymorphism (SNP) in exon 2 at the SRSF2 binding domain, which results in the skipping of exon 2. Thus, CD33 exists in 2 main isoforms regulated in part by presence of the SNP, as either a full-length (FL) transcript or a truncated variant missing exon 2 (DE2). Exon 2 encodes the IgV domain, which serves as the epitope for many diagnostic and therapeutic antibodies, including GO. The DE2 variant would encode a protein devoid of antibody binding site, thus would be expected to lack therapeutic efficacy. This hypothesis was confirmed in the COG AAML0531 study, where GO was found to have pronounced therapeutic benefit in patients with CC genotype (FL CD33), but lacked efficacy among those with TT genotype(DE2 CD33)(Lamba et al, JCO 2017). In order to confirm efficacy of GO against FL vs. DE2 transcripts, we evaluated the cytotoxic effect of GO in cell lines engineered to express the FL and DE2 isoforms. In addition, we evaluated the efficacy of 2 other CD33-directed ADCs, SGN-33A and IMGN-79 in these cell lines, given that the epitopes for these ADCs are unknown.
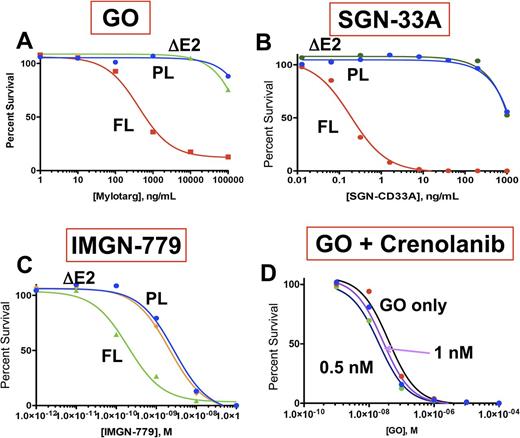
We engineered the two CD33 transcript variants, FL and DE2, and expressed them in the CD33-negative cell line Ba/F3. Expression of CD33 in parental or CD33 transduced cells was confirmed by multi-dimensional flow cytometry and RT-PCR. Cells were incubated with varying doses of GO, SGN-33 or IMGN-779 for 72 hours and then underwent evaluation of cell viability by CellTiter-Glo. In cells expressing FL CD33, GO demonstrated significant efficacy, causing cellular apoptosis with an IC50 of 422 ng/mL. However, cells expressing the DE2 variant showed no response to GO, with a similar profile to CD33-negative parental cells (Figure 1A). Evaluation of the efficacy of SGN-33A and IMGN-779 showed similar efficacy profiles, with potent pro-apoptotic effects in cells with FL transcripts with an IC50 of 0.92 ng/mL with SGN-33 and 0.2 nM with IMGN-779. Similar to GO, neither SGN-33A, nor IMGN-779 showed any efficacy in cells expressing the DE2 variant or parental CD33-negative cells, thus demonstrating that the paratope for both ADCs is significantly more effective at recognizing FL CD33 (Figure 1B and 1C).
The FLT3 /ITD-positive AML cell line MV4;11 is CT genotype, thus expresses both FL and DE2 isoforms. The COG and ALFA clinical trials have shown that GO has pronounced efficacy in FLT3 /ITD+ patients, we therefore inquired whether efficacy of GO might be potentiated in FLT3 /ITD AML. MV4;11 cells was exposed to varying doses of GO in the presence or absence of sub-IC50 doses of FLT3 inhibitor crenolanib (0.5 and 1.0 nM) and cell survival was assessed by CellTiterGlo. Evaluation of GO plus crenolanib demonstrated an IC50 of 42 ng/mL for GO alone vs. 32 ng/mL in the presence of 0.5nM and 26 ng/mL in the presence of 1 nM crenolanib (Figure 1D), demonstrating that GO efficacy can be enhanced in combination with TKIs in FLT3 /ITD AML.
Cell surface CD33 is present in both long and short isoform, with expression regulated in part by splicing. We show that surface expression of FL CD33 is necessary for therapeutic efficacy of the most promising CD33-directed ADCs that are currently available for use. We further provide data that CD33 targeted agents can be effectively combined with FLT3 directed TKIs allowing for enhanced efficacy at lower doses and potentially lower toxicity. This work provides biologic rationale for more targeted approach to treating patients with CD33-directed ADCs. Future investigations should focus on optimizing CD33-directed therapy through determination of thresholds of biologic activity for specific agents and the use of combination therapies for patients based on the leukemic profile, thus improving treatment outcomes and avoiding unnecessary toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal